Every element can exist in three states:
as a liquid, as a solid and as a vapor, which mostly depend
on it's temperature. This applies to water, too. So, water can
be found as ice, water and steam. If water is cooled down below
0 degrees Celsius (32 Fahrenheit), it becomes ice, and if heated
above 100 degrees Celsius (212 Fahrenheit), it becomes steam.
The temperature, at which a substance changes it state from liquid
to vapor is called a boiling point, and it is different for different
substances. This difference can be used to separate substances,
and as such can be used for water purification.
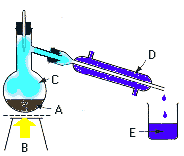 |
| The
process is relatively simple: |
| a) |
the dirty water
is heated |
| b) |
to the boiling
point and thus vaporizes |
| c) |
(becomes steam),
while other substances remain in solid state, in boiler.
Steam is then directed into a cooler |
| d) |
where it cools
down and returns to liquid water |
| e) |
and the end
result is a water, purified of additional substances
found in it before distillation. |
|
Distillation is an effective process
and, what's more important, it can be done with a lot of improvisation.
You can heat water with whatever is at hand: fire, electricity,
or whatever. You can use almost anything that holds water
for a boiler, as long as you can direct the steam into a cooler.
A cooler can be a long piece of copper tubing bent into a spiral.
All you need is something that will just cool the steam down.
In a worst case scenario, you can distill water with an ordinary
household pot and two pot lids. Boil water in a pot covered with
the first lid. After a while, you'll see that the water
in the pot vaporizes, and condenses on the lid (this is distilled
water). Now replace the lid with the second lid, and turn the
first one vertically, so that all condensed water collects at
one point, and then pour it into a cup. Meanwhile, more distilled
water condenses on the second pot lid, so just repeat the above
steps again... until you have a full cup.
Distillation will remove from water
almost anything, even heavy metals, poisons, bacteria and viruses.
However, it does not remove substances that have boiling points
at a lower temperature than water. Some of these substances are
oils, petroleum, alcohol and similar substances, which in most
cases don't mix with water. Also, remember that substances
removed from water remain in the boiler, so you'll need to clean
it up every once in awhile.
Distilled water can be used directly
and does not need to be boiled again. As it is already hot, you
can use it to prepare tea, or similar drinks.
|